By Guest Blogger, Matt Gunther
This month, Gunther discusses what is arguably the most glamourous organism in the scientific world: algae. The bane of pond-owners with clogged-up fiter pumps, algae has some very exotic properties, which make it the perfect candidate for sucking up radioactive contamination in the world's rivers and oceans. They also seem to get a kick out of smothering people in foam?!
------------------------------------
 |
| Clearly this foam party got out of hand?! |
Say the word 'algae' to anyone and I'd imagine the descriptions of 'scum', 'crap' and 'yuccy slime' will come to mind. Although they're an annoyance to anyone with a pond full of over-priced fish, these organisms' properties can result in some very bizarre natural phenomena occurring; including what has befallen the young chap in the image above.
The tidal wave of foam chasing the boy down is believed to be as a result of algae, which has not received enough energy from the Sun. These algal colonies of the so-called Phaeocystis Globosa family eventually die and - like all organic matter - decay. Swept up by the wind, the organic detritus is consequently churned up at shorelines, thereby producing the thick foam you see before you - almost like detergent in a washing machine. Although, the image above proves to be rather amusing, I suppose you're wondering why in God's name I'm bringing it up. Well, this algae thrives, as it were, on electromagnetic radiation from the Sun in the form of light.
In a similar fashion to light, algae also has the ability to absorb and consume other sources of radiation, as well as radioactive compounds. For instance, many bio-organisms have the ability to absorb UV and re-emit radiation at a different wavelength. This process is commonly known as fluorescence. Whilst providing us with some very pretty pictures, this mechanism can provide us with detailed information on the structure and chemical composition of marine biotic life, using techniques such as fluorescence spectroscopy.
Particular algal species, as I've mentioned, also have the potential to absorb radioactive isotopes. This may prove to be of vital importance in the nuclear industry, whereby possible contamination in water supplies are hard problems to tackle without damaging a local ecosystem.
The Fukushima nuclear plant in Japan is currently attempting to tackle this very problem and they're dealing with a very nasty radioisotope called Strontium-90. Known as the "bone seeker", strontium has a lot of very similar biochemical characteristics to the "friendly-neighbourhood" element of the periodic table: calcium. Like calcium, if strontium-90 is ingested through the consumption of liquid, the vast majority of the radioactive atoms are absorbed by your teeth and bone marrow. With strontium (a strong beta emitter) also having a long half-life relative to the average human lifespan, an ingestion of it may lead to the contraction of cancer in later life and other serious health problems.
Unfortunately for the chaps in Japan, there is approximately ten billion times as much calcium as there is strontium emitted during a typical nuclear plant leak into local rivers/oceans. Due to the similar characteristics of both elements, it's very hard to remove one without removing the other. Cue C. moniliferum.
 |
| Algae fluorescing on a dive in the Red Sea. |
Particular algal species, as I've mentioned, also have the potential to absorb radioactive isotopes. This may prove to be of vital importance in the nuclear industry, whereby possible contamination in water supplies are hard problems to tackle without damaging a local ecosystem.
 |
| 1950s nuclear paranoia led to people checking for strontium in their milk.......which was in a thick-walled glass bottle.... Hhhmmm (baby produces a sceptical face) |
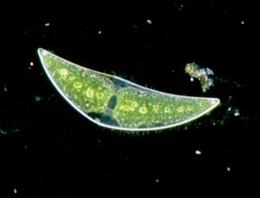 |
| C. Moniliferum. The banana-shaped algae with a penchant for chomping on strontium. |
Not content with the ability to fluoresce, this superhero species loves to collect barium. As strontium is halfway between calcium and barium in terms of size, the algae collects strontium and crystallises it out, forming an easy-to-remove solid substance containing barium enriched with strontium [1]. Furthermore, calcium is different enough, in terms of it's chemical size, to not be absorbed by the moniliferum species [1]. It is incredible a natural organism can be so efficient in removing some nasty contaminants from the sea and its all the more staggering due to the elegant simplicity of the process..... Not forgetting its cheap too!
There are many more examples of using bio-organisms to remove contamination from the soil, as well as from the air and oceans. It is a branch of science which is fancifully known as phytoremediation. A low-cost and effective way of repairing polluted areas, phytoremediation has been used to accumulate and degrade contamination surrounding Chernobyl, as well as being used in the coal mining industry to treat discharges.
What is truly wonderful about this method of removing contaminants, is that we are using the power of nature in order to solve a man-made problem. It is truly one of the reasons why I find science such a fascinating subject. No matter how technology advances and continues to dazzle us in its complexity, you just can not beat the beauty, dexterity and simplicity of nature.
REFERENCES
[1] R. Lovett, "Algae Holds Promise For Nuclear Clean-Up" (Nature, 2011), http://www.nature.com/news/2011/110330/full/news.2011.195.html
Images taken from; http://cfb.unh.edu/phycokey/Choices/Prymnesiophyceae/PHAEOCYSTIS/Phaeocystis_Image_page.html, http://www.dailymail.co.uk/sciencetech/article-2165450/The-red-green-blue-yellow-sea-Neon-lights-turn-the-Red-Sea-sponge-disco.html, http://nuclearhistory.wordpress.com/2010/10/26/where-has-all-the-strontium-gone/, http://www.nature.com/news/2011/110330/full/news.2011.195.html
